-
 Revolutionizing IBD treatment!
Revolutionizing IBD treatment! -
 Personalized electronic therapeutic capsulefor Inflammatory Bowel Diseases (IBD)
Personalized electronic therapeutic capsulefor Inflammatory Bowel Diseases (IBD)
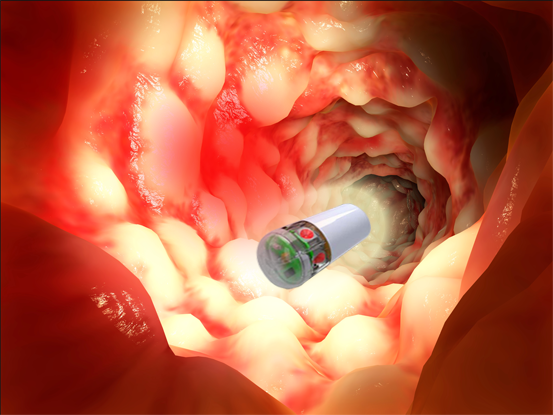
Overview
PhotoPill Medical is harnessing the power of light to assist patients suffering from Inflammatory Bowel Disease (IBD).
PhotoPill Medical develops a revolutionary disposable swallowable electronic capsule for treating Crohn’s disease, by implementing Photo-Bio-Modulation treatment (PBMt) and gut microbiota modulating light protocols as it travels through the intestine. PhotoPill’s therapeutic capsule – the IBD-Cap™ was designated by the FDA as a “Breakthrough Device” for the treatment of Crohn’s Disease flare-ups that are non-responding to other treatments. The IBD-Cap™ is personalized for each patient, based on disease location and individual peristaltic rate. The IBD-Cap™ unique disruptive technology would be a first of its kind for treating intestinal inflammation, for mucosal healing and for gut bacteria related disorders. This would be a much safer treatment comparing to the currently existing leading medications, an easy to use and cost-effective solution for a huge market and a painful problem.
PhotoPill Medical is motivated by the need of IBD patients for better and safer treatments, and aims to help patients cope with the challenges they are facing on daily basis.
The company
PhotoPill Medical was founded by Sharon Ben-Yehuda, a biochemist and a Crohn’s Disease patient, that out of her own pain and experience, was eager to find a way to assist IBD patients and reduce the significant difficulties and distress associated with disease flare-ups. PhotoPill’s goal is to provide safer, cost-effective and efficacious IBD treatments. PhotoPill has demonstrated excellent pre-clinical results and initial clinical efficacy results in IBD Proctitis patients, using the ProctCare™ – PhotoPill’s dedicated rectal treatment device. The company is now starting a multi-center clinical study with Crohn’s Disease patients using the IBD-Cap™. PhotoPill Medical has strong IP and strong and experienced management team.
About IBD
Inflammatory Bowel Disease (IBD) is a set of chronic diseases characterized by intense inflammatory process and ulcerations involving the intestine walls. It is estimated that there are tens of million IBD patients worldwide, of which about half are Crohn’s Disease patients. Currently, there is no cure for IBD, and daily use of medications is the gold standard of treatment to prolong remission periods between flare-ups; episodes that are related to sever pain, diarrhea, bloody stool, fatigue, anemia, and also extraintestinal manifestations; all may require hospitalization.
IBD medications range from 5-ASA anti-inflammatory drugs, antibiotics, steroids, and up to Immunomodulators and Biologic agents. These medications are mostly systemic and have the potential for significant side effects. The PhotoPill™ treatment is not systemic and involves minimal side effects.
To find out more about IBD, use the following links:
www.crohnscolitisfoundation.org
www.efcca.org

Overview
PhotoPill Medical is harnessing the power of light to assist patients suffering from Inflammatory Bowel Disease (IBD).
PhotoPill Medical develops a revolutionary disposable swallowable electronic capsule for treating Crohn’s disease, by implementing Photo-Bio-Modulation treatment (PBMt) and gut microbiota modulating light protocols as it travels through the intestine. PhotoPill’s therapeutic capsule – the IBD-Cap™ was designated by the FDA as a “Breakthrough Device” for the treatment of Crohn’s Disease flare-ups that are non-responding to other treatments. The IBD-Cap™ is personalized for each patient, based on disease location and individual peristaltic rate. The IBD-Cap™ unique disruptive technology would be a first of its kind for treating intestinal inflammation, for mucosal healing and for gut bacteria related disorders. This would be a much safer treatment comparing to the currently existing leading medications, an easy to use and cost-effective solution for a huge market and a painful problem.
PhotoPill Medical is motivated by the need of IBD patients for better and safer treatments, and aims to help patients cope with the challenges they are facing on daily basis.
The company
PhotoPill Medical was founded by Sharon Ben-Yehuda, a biochemist and a Crohn’s Disease patient, that out of her own pain and experience, was eager to find a way to assist IBD patients and reduce the significant difficulties and distress associated with disease flare-ups. PhotoPill’s goal is to provide safer, cost-effective and efficacious IBD treatments. PhotoPill has demonstrated excellent pre-clinical results and initial clinical efficacy results in IBD Proctitis patients, using the ProctCare™ – PhotoPill’s dedicated rectal treatment device. The company is now starting a multi-center clinical study with Crohn’s Disease patients using the IBD-Cap™. PhotoPill Medical has strong IP and strong and experienced management team.

The science
Photo-Bio-Modulation treatment (PBMt), which is also referred to as Low Level Light Therapy (LLLT), is the therapeutic use of non-thermal non-ionizing specific wavelengths (mainly Red and Near Infrared) for treating inflammatory, wound, pain and COVID-19 pneumonia conditions. PBMt has demonstrated a therapeutic effect on inflammation and ulceration seen in several indications that involves mucosal tissue.
PBMt meets the definition of medical necessity for prevention of oral mucositis in members undergoing cancer treatment associated with increased risk of oral mucositis, and was adopted by The National Institute for Health and Care Excellence (NICE) in the UK, by the International Society of Oral Oncology (ISOO) and by the Multinational Association of Supportive Care in Cancer (MASCC). Additionally, BlueCross BlueShield adopted PBM for OM (Sep 2019) as an experimental treatment modality.
There are publications about other mucosal inflammatory conditions that showed clinical improvement with PBM treatment, such as: Oral Crohn’s Disease, healing of intestinal anastomoses, Payer’s Patches and lately several publications about PBMt for COVID-19 pneumonia.
Inflammatory Bowel Disease (IBD) is an immune-mediated chronic disease characterized with painful and disabling cycles of flare-ups and remissions. Research on IBD has identified disrupted immune responses in the gastrointestinal mucosa and putative disruptions in the gut microbiome as causative agents.
The proposed mechanisms of action for PBMt when used as a therapeutic modality for IBD, are in two aspects that are closely related to the etiology and pathophysiology of IBD. One is the metabolomic aspect, where light is absorbed by the chromophore Cytochrome C Oxidase in the Mitochondria, and the second is the Microbiomic aspect, where light influences the gut bacteria population. There are publications demonstrating the effect of PBMt on epithelial barrier integrity. Cytochrome c oxidase (Cco) is proposed to be the primary photo-acceptor for the Red / Near-Infra-Red light range in mammalian cells. The reception of Red-NIR light by Cco triggers a chain of activities which increases ATP synthesis, increased RNA and protein synthesis, increase oxygen consumption, lymphatic drainage, mitochondrial membrane potential and more. Other downstream processes influence anti-and-proinflammatory cytokines (such as TNFa, IL-1, IL-6), macrophages phenotype (M1to M2), cell migration, apoptosis and more.
The science
Photo-Bio-Modulation treatment (PBMt), which is also referred to as Low Level Light Therapy (LLLT), is the therapeutic use of non-thermal non-ionizing specific wavelengths (mainly Red and Near Infrared) for treating inflammatory, wound, pain and COVID-19 pneumonia conditions. PBMt has demonstrated a therapeutic effect on inflammation and ulceration seen in several indications that involves mucosal tissue.
PBMt meets the definition of medical necessity for prevention of oral mucositis in members undergoing cancer treatment associated with increased risk of oral mucositis, and was adopted by The National Institute for Health and Care Excellence (NICE) in the UK, by the International Society of Oral Oncology (ISOO) and by the Multinational Association of Supportive Care in Cancer (MASCC). Additionally, BlueCross BlueShield adopted PBM for OM (Sep 2019) as an experimental treatment modality.
There are publications about other mucosal inflammatory conditions that showed clinical improvement with PBM treatment, such as: Oral Crohn’s Disease, healing of intestinal anastomoses, Payer’s Patches and lately several publications about PBMt for COVID-19 pneumonia.
Inflammatory Bowel Disease (IBD) is an immune-mediated chronic disease characterized with painful and disabling cycles of flare-ups and remissions. Research on IBD has identified disrupted immune responses in the gastrointestinal mucosa and putative disruptions in the gut microbiome as causative agents.
The proposed mechanisms of action for PBMt when used as a therapeutic modality for IBD, are in two aspects that are closely related to the etiology and pathophysiology of IBD. One is the metabolomic aspect, where light is absorbed by the chromophore Cytochrome C Oxidase in the Mitochondria, and the second is the Microbiomic aspect, where light influences the gut bacteria population. There are publications demonstrating the effect of PBMt on epithelial barrier integrity. Cytochrome c oxidase (Cco) is proposed to be the primary photo-acceptor for the Red / Near-Infra-Red light range in mammalian cells. The reception of Red-NIR light by Cco triggers a chain of activities which increases ATP synthesis, increased RNA and protein synthesis, increase oxygen consumption, lymphatic drainage, mitochondrial membrane potential and more. Other downstream processes influence anti-and-proinflammatory cytokines (such as TNFa, IL-1, IL-6), macrophages phenotype (M1to M2), cell migration, apoptosis and more.

The PhotoPill™ technology
Current treatments for IBD are far from ideal, and given the potential of PBMt to influence patient’s immune system as well as tissue rejuvenation and gut microbiota population, we have developed a swallowable autonomous vehicle – a pill-size electronic autonomous capsule, equipped with a novel localization technology and a proprietary software, which allows the capsule to be activated at the inflammation area, delivering PBMt directly to the lesion and to the lumen, illuminating anti-inflammatory, tissue healing light protocols, as well as microbiota manipulating light protocols.
PhotoPill’s IBD-Cap™ is pre-programed to deliver a topical targeted minimal side effects PBM treatment, taking into consideration the individual disease location, and adjusts the dose of treatment to the peristaltic rate and capsule’s speed at any given moment. The capsule does not require any external belt or outside stirring, as it is completely autonomous.
IBD-Cap™ is a unique and novel platform for delivering PBM treatment into the intestine in a topical manner, targeted directly to the tissue and to the lumen. It opens the opportunity to make a therapeutic first of its kind impact on the complex ecosystem in the gut and the synergic relationship that exist between the mucosal immune system and gut microbiome.
IBD-Cap™ technology that enables the pre-programing of the capsule to identify its arrival to the desired location, with accordance to the patient’s individual peristaltic rate and disease location, is a breakthrough technology that addresses the most advanced and latest scientific discoveries and insights of IBD pathophysiology and of new therapeutic approaches, that will enable a more efficacious treatment, that not only inhibits specific cytokines in the inflammatory reaction, and not only changes specific bacteria in the gut, but actually influences both of these systems and their subsequent very delicate and complex relationship, which is so fragile and disrupted in IBD conditions.
The PhotoPill™ treatment aims at broadening the therapeutic armamentarium that physicians have, when facing this debilitating chronic disease, using a novel technology and approach that was not available until now.
Being a non-pharmacological treatment modality, is another novelty of the PhotoPill capsule treatment – enabling a combined therapy with other medications, a supplement or substitute to current medications in cases of non-respondence or loss of response over time. IBD-Cap™ may also be a solution for adverse reactions caused by medications, or for patients that are reluctant to use existing pharmacological treatments.
The PhotoPill™ technology
Current treatments for IBD are far from ideal, and given the potential of PBMt to influence patient’s immune system as well as tissue rejuvenation and gut microbiota population, we have developed a swallowable autonomous vehicle – a pill-size electronic autonomous capsule, equipped with a novel localization technology and a proprietary software, which allows the capsule to be activated at the inflammation area, delivering PBMt directly to the lesion and to the lumen, illuminating anti-inflammatory, tissue healing light protocols, as well as microbiota manipulating light protocols.

PhotoPill’s IBD-Cap™ is pre-programed to deliver a topical targeted minimal side effects PBM treatment, taking into consideration the individual disease location, and adjusts the dose of treatment to the peristaltic rate and capsule’s speed at any given moment. The capsule does not require any external belt or outside stirring, as it is completely autonomous.IBD-Cap™ is a unique and novel platform for delivering PBM treatment into the intestine in a topical manner, targeted directly to the tissue and to the lumen. It opens the opportunity to make a therapeutic first of its kind impact on the complex ecosystem in the gut and the synergic relationship that exist between the mucosal immune system and gut microbiome.
IBD-Cap™ technology that enables the pre-programing of the capsule to identify its arrival to the desired location, with accordance to the patient’s individual peristaltic rate and disease location, is a breakthrough technology that addresses the most advanced and latest scientific discoveries and insights of IBD pathophysiology and of new therapeutic approaches, that will enable a more efficacious treatment, that not only inhibits specific cytokines in the inflammatory reaction, and not only changes specific bacteria in the gut, but actually influences both of these systems and their subsequent very delicate and complex relationship, which is so fragile and disrupted in IBD conditions.
The PhotoPill™ treatment aims at broadening the therapeutic armamentarium that physicians have, when facing this debilitating chronic disease, using a novel technology and approach that was not available until now.
Being a non-pharmacological treatment modality, is another novelty of the PhotoPill capsule treatment – enabling a combined therapy with other medications, or a supplement or substitute to current medications in cases of non-respondence or loss of response over time. IBD-Cap™ may also be a solution for adverse reactions caused by medications, or for patients that are reluctant to use existing pharmacological treatments.
The PhotoPill™ capsule
Light based targeted treatment
Autonomous
Intestinal navigation capability
Adjusted to peristaltic rate & Disease location
Affordable
Safe
Team

Sharon Ben Yehuda
Co-Founder
Sharon is an experienced business development executive in the medical device field, with years of experience in setting up and managing medical device start-ups. Sharon holds BSc in Biochemistry and clinical nutrition (graduated summa cum Laude) from the Hebrew University in Jerusalem and is a certified CRO. In addition to being a Crohn’s disease patient, Sharon has experience in clinical work and working with IBD patients and is a member of the CCFI (Crohn’s & Colitis Foundation of Israel) scientific committee.

Rami Ben Yehuda
Co-Founder/CEO
Ram has over 22 year of experience in the field of medical devices, extending from Being VP R&D and VP sales and marketing to COO and CEO. Ram has held key positions in several International medical device companies. Ram holds a B.Sc. in Electronics Engineering, and a M.Sc. in Biomedical Engineering and Management Studies from Tel Aviv University.

Semion Khait
R&D Leader
For the past 19 years, Semion held leading R&D positions in Given Imaging, later Covedian and Medtronic, where he led the development and research activities of the hardware capsule endoscopy development. Semion is a Technical Fellow and Director, Capsule Electronics at Medtronic, MITG, ET, GIH, Given Imaging. Semion’s experience and knowledge in capsule development is unique in the world and is a global leader in capsule development in Medtronic international.

Dr. Yuval Binur
Chairman
DR. Binur has over 30 years of worldwide venture capital and investment experience. Dr. Binur co-founded Accelerated Technologies, Inc. – a highly differentiated investment firm focused on medical technologies and specifically on cardiovascular. Prior to that, Dr. Binur was a founding partner of Medica Venture Partners, Israel’s first dedicated life sciences and medical technologies venture fund. Prior to that, Dr. Binur was a member and manager of Adler & Co., a large New York based venture capital firm. Throughout his carrier in venture capital, Dr. Binur was involved with major M&A transactions, fund raising and IPOs totaling approx. US$2 Billion.

Prof. David Rubin
Clinical advisory board
Prof. Rubin is Professor of Medicine at University of Chicago, Section Chief, Gastroenterology, Hepatology and Nutrition, Co-Director, Digestive Diseases Center. Areas of Expertise: Inflammatory Bowel Disease (IBD), Crohn’s Disease, Ulcerative Colitis, Inflammatory Bowel Disease Surgery, Colon Cancer Prevention Chromoendoscopy, Genetics, Medical Ethics.
Prof. Rubin studies novel therapies for Crohn’s disease and ulcerative colitis, colon cancer prevention, and clinical medical ethics.

Prof. Yehuda Chowers
Clinical advisory board
Prof. Chowers is Director of the Gastroenterology Institute at Rambam Health Care Campus, as well as an Associate Professor at the Rappaport Faculty of Medicine at the Technion. In addition, he has been a Principle Investigator and member of the Clinical Research Institute at Rambam (CRIR) since 2008.Prof. Chowers’ clinical specialties are in the field of chronic inflammatory bowel diseases, including Crohn’s disease, ulcerative colitis, and radiation-induced bowel injury.
Contact Us
